BRINGING EXPERTS AND INNOVATION TOGETHER
It is D’Silva’s mission to ensure that medical devices are designed and developed in a safe and efficient way

Working with Start-up Companies

Support from `Concept´ to ´Production`

Global Market Support

Regulatory Strategy - E.U & FDA

ISO 13485 Quality Management System (QMS) Creation & Support

EU MDR (PRRC)

Clinical Study Support
Authorized Representative & Importing Activities for Non-E.U Clients
Particulate testing according to ISO laboratory Standards
Biocompatibility & Sterilization Support
D´Silva medical device consulting endeavors in bringing high-level expertise and individual experts to your needs. Equipped with over 16 years of healthcare experience, D´Silva supports all stages of medical device development for access to the European, Asian, Latin American, Canadian, and U.S Markets. Work with D´Silva medical device consulting to ensure your product is safe, effective, compliant, and a benefit to healthcare professionals and patients. Benefit from the wide range of expertise to bring your product or idea to market in a lean and cost-effective way.
Medical Device Consulting in the following Areas


Production & Manufacturing
Currently working with a group of established Contract and Legal Manufacturers worldwide the option to introduce your product at all stages of its development lifecycle is possible.

Quality Management System
D’Silva and his team have a significant level of QMS experience to support any aspect of your requirements. This experience extends to various global markets including Brazil, Japan, Hong Kong & Argentina.

Clinical Trials & Clinical Evaluations
The requirements for clinical evidence within the CE conformity assessment process of medical devices have further increased. Under the European Medical Device Regulation (2017/745) (MDR), there are important new requirements for pre-market and post-market clinical investigations.

Regulatory Affairs
D’Silva and his team have a significant level of regulatory experience to support a wide range of regulatory requirements. This experience extends to various global markets including Brazil, Japan, Hong Kong, Canada, Switzerland, UK & Argentina.

Prototyping, Research & Development
Through the network of D’Silva’s strategic partners, a wide range of experience in product design and development is available to support your idea from early-stage R&D concepts to a functional prototype.

Biocompatibility & Sterilization Support
In addition to the skill set of biocompatibility, the team is also experienced in the areas of Particulate testing, Sterilization, and Cleanroom Expertise (Set-up and monitoring).
Imaging Modalities
A wide range of imaging options can be provided to validate manufacturing processes, process validations, requests from notified bodies or regulatory agencies. Please use the Contact Us form for further information.
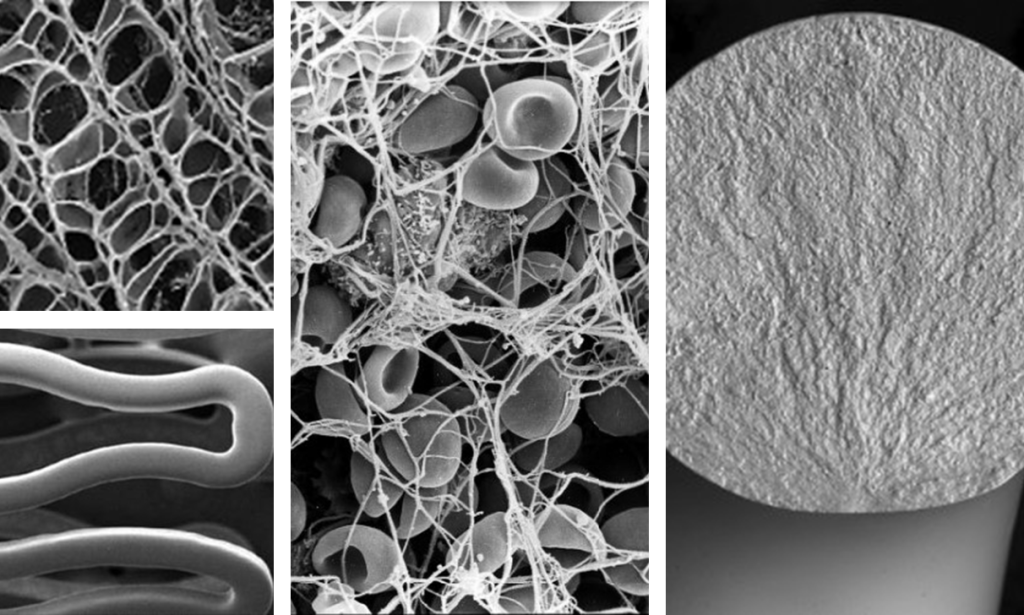
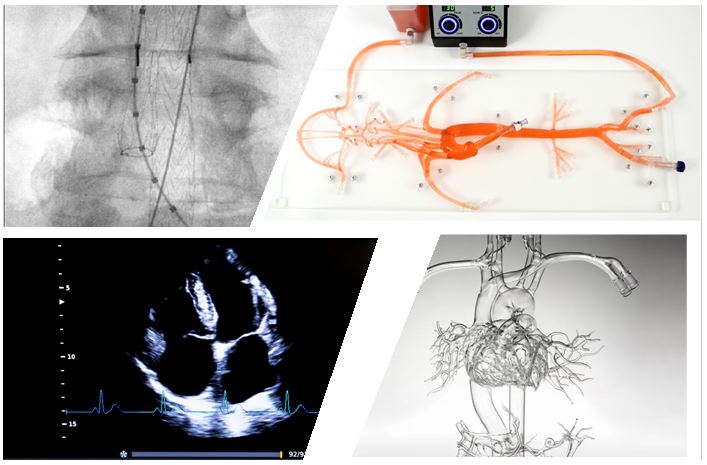
Usability & Simulated Use
Bench testing, Simulated use, and Usability experiments can be performed in our selected laboratories to test your device through its developmental life cycle. Animal & Cadaver experiments are also available upon request.
